Electrolytes are essential minerals that carry an electric charge when dissolved in body fluids, playing a critical role in everything from nerve function to hydration.
While you might associate them with sports drinks, their importance extends far beyond athletic performance.
In fact, an imbalance in these vital particles can affect nearly every system in your body, and studies show electrolyte disorders are surprisingly common, affecting up to 64% of critically ill patients upon hospital admission.
Understanding electrolytes is fundamental to managing your overall health.
This comprehensive guide will break down the science behind these charged minerals, exploring their seven core functions, the signs of an imbalance, and how to maintain healthy levels through diet and proper hydration.
We’ll delve into the specific needs of different populations, from endurance athletes to older adults, and provide evidence-based advice to help you take charge of your body’s delicate electrical system.
In This Article
What Exactly Are Electrolytes? A Simple Definition
At its core, the term electrolyte refers to any substance that produces an electrically conducting solution when dissolved in a polar solvent, such as water.
In nutrition and medicine, electrolytes are essential minerals—like sodium, potassium, and calcium—that are found in your blood, sweat, and urine.
When these minerals dissolve, they separate into positively or negatively charged particles called ions.
An adult’s body is about 60% water, which means these charged ions are present in nearly every fluid and cell.
This electrical potential is what allows them to conduct nerve impulses, stimulate muscle contractions, and regulate countless other critical bodily processes.
The Science Behind the Charge: Ions, Cations, and Anions
To understand how electrolytes work, think of salt (sodium chloride) dissolving in water.
The salt molecule splits into two ions: a positively charged sodium ion (Na+) and a negatively charged chloride ion (Cl-). These charged particles are the electrolytes.
- Ions: Atoms or molecules that have gained or lost one or more electrons, giving them a net electrical charge.
- Cations: Ions with a positive charge (e.g., Sodium, Potassium, Calcium, Magnesium).
- Anions: Ions with a negative charge (e.g., Chloride, Bicarbonate, Phosphate).
This electrical opposition between cations and anions is what allows your body to send signals and perform work.
As Cleveland Clinic explains, electricity jumps between these ions, not the water molecules themselves, enabling the complex communication network that keeps you alive.
More Than Just Minerals: How They Work in Body Fluids
Your body works tirelessly to maintain a precise balance of these electrolytes in your intracellular fluid (inside the cells) and extracellular fluid (outside the cells, including blood plasma).
This balance is crucial for fluid regulation and cellular function.
Your kidneys are the primary regulators, filtering excess electrolytes from the blood and excreting them in urine.
You also lose electrolytes through sweat, which is why rehydration after intense activity is so important.
Why Are Electrolytes Essential for Your Body? (The 7 Core Functions)
Electrolytes are not just passive particles, they are active participants in the fundamental processes that sustain life.
Their electrical charge empowers them to perform a variety of vital functions throughout the body.
1. Powering Your Nervous System
Your brain communicates with the rest of your body by sending electrical signals, known as nerve impulses, through nerve cells.
These signals are generated by changes in the electrical charge across the nerve cell membrane.
The electrolyte sodium is the primary driver of this process.
When a nerve cell is stimulated, channels open to allow sodium ions to rush into the cell, creating an electrical spark that travels down the length of the nerve.
2. Enabling Muscle Contraction and Relaxation
Every movement you make, from a heartbeat to lifting a weight, depends on electrolytes.
As described in health resources, calcium ions are required for muscle fibers to slide together, causing a muscle to contract.
After contraction, magnesium steps in to help the fibers slide apart, allowing the muscle to relax.
An imbalance in either can lead to muscle weakness, spasms, or cramps.
3. Maintaining Optimal Hydration (The Role of Osmosis)
Electrolytes, particularly sodium, are key to maintaining the correct amount of water inside and outside your cells.
They do this through a process called osmosis, where water moves across a cell membrane from an area of lower solute concentration to an area of higher concentration.
This ensures cells don’t shrivel from dehydration or burst from being too full.
4. Regulating Your Body’s pH Balance
Your body must maintain a very specific pH level to function correctly.
For example, your blood pH must stay within a narrow range of 7.35 to 7.45.
Electrolytes like bicarbonate and phosphate act as chemical buffers, helping to neutralize acids and bases to keep your internal environment stable.
Deviations from this range can impair metabolic processes and lead to serious illness.
5. Moving Nutrients and Waste
Electrolytes help transport nutrients into your cells and move metabolic waste products out.
For instance, sodium helps cells absorb essential nutrients like glucose and amino acids.
This process is vital for energy production and cellular repair.
6. Supporting Cardiovascular Health
The steady rhythm of your heart is controlled by electrical impulses, which are entirely dependent on the movement of electrolytes like potassium, calcium, and magnesium.
The American Heart Association emphasizes the importance of electrolyte balance for cardiovascular health, noting that imbalances can lead to arrhythmias (irregular heartbeats) and affect blood pressure.
7. Building and Maintaining Strong Bones
While not their primary electrical function, minerals that act as electrolytes are also structural components of your body.
Calcium and phosphate are the main building blocks of bones and teeth, providing the strength and integrity of your skeletal system.
The Main Types of Electrolytes in Your Body
Your body utilizes several key minerals as electrolytes, each with a unique role. Understanding what each one does helps clarify why a balanced intake is so crucial.
| Electrolyte (Ion) | Primary Functions | Imbalance Name (Low) | Imbalance Name (High) |
|---|---|---|---|
| Sodium (Na+) | Maintains fluid balance, nerve function, muscle contraction, nutrient absorption. | Hyponatremia | Hypernatremia |
| Potassium (K+) | Regulates heart rhythm, muscle function, nerve signals, works with sodium. | Hypokalemia | Hyperkalemia |
| Calcium (Ca2+) | Essential for muscle contraction, nerve signaling, blood clotting, bone/teeth health. | Hypocalcemia | Hypercalcemia |
| Magnesium (Mg2+) | Aids muscle relaxation, nerve function, energy production, DNA synthesis, heart rhythm. | Hypomagnesemia | Hypermagnesemia |
| Chloride (Cl-) | Maintains fluid balance, blood pressure, and body’s pH level. | Hypochloremia | Hyperchloremia |
| Phosphate (PO4³⁻) | Forms bones/teeth, helps cells produce and use energy (ATP), component of DNA. | Hypophosphatemia | Hyperphosphatemia |
| Bicarbonate (HCO3-) | Key buffer that maintains the body’s acid-base (pH) balance. | Acidosis | Alkalosis |
Source: Information compiled from MedlinePlus and Cleveland Clinic.
What Is an Electrolyte Imbalance?
An electrolyte imbalance occurs when the level of one or more electrolytes in your blood becomes too high or too low.
Because these minerals are involved in so many vital functions, even a slight disturbance can have noticeable effects on your health.
In severe cases, imbalances can be life-threatening.
Understanding “Hyper” (Too Much) and “Hypo” (Too Little)
Medical terms for electrolyte imbalances use prefixes to indicate the nature of the problem:
- Hyper- means “too much”. For example, hyperkalemia is an excess of potassium.
- Hypo- means “too little”. For example, hyponatremia is a deficiency of sodium.
Common Causes: From Dehydration to Medical Conditions
The most frequent cause of an electrolyte imbalance is a change in your body’s water levels. According to MedlinePlus, common causes include:
- Dehydration from severe vomiting, diarrhea, or heavy sweating.
- Overhydration (drinking too much water without replacing electrolytes).
- Kidney disease, which impairs the ability to filter and regulate electrolytes.
- Heart, liver, or endocrine disorders.
- Certain medications, such as diuretics (“water pills”), ACE inhibitors, and some antibiotics.
- Malnutrition or eating disorders.
- Intense, prolonged exercise, especially in the heat.
Symptoms to Watch For: When to See a Doctor
Mild imbalances may not produce any symptoms. However, as the imbalance becomes more severe, you might experience:
- Fatigue or lethargy
- Muscle weakness, spasms, or cramps
- Nausea and vomiting
- Headache
- Confusion or irritability
- Irregular or fast heart rate (palpitations)
If you suspect you have an electrolyte imbalance, especially if you have an underlying medical condition, it is crucial to speak with your doctor. Do not attempt to self-treat a severe imbalance.
A Deep Dive into Specific Electrolyte Imbalances
Let’s explore the most common imbalances in more detail, including their specific causes and symptoms.
Sodium Disorders: Hyponatremia and Hypernatremia
Sodium is the most abundant electrolyte in the body and is critical for fluid balance.
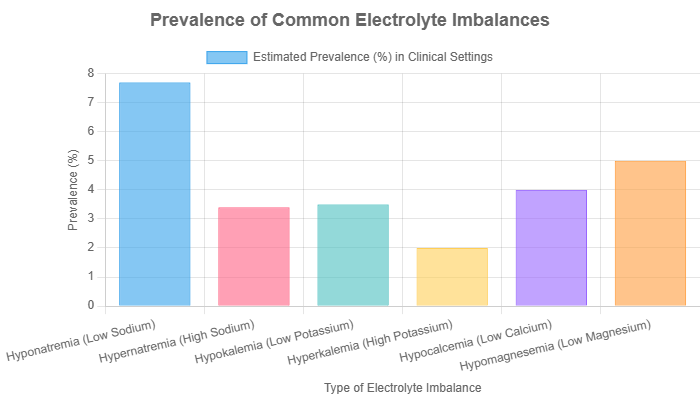
Hyponatremia (low sodium, typically <135 mEq/L) is the most common electrolyte disorder found in clinical practice. It’s often caused by excessive water intake, heart failure, kidney disease, or certain diuretics. Symptoms can range from nausea and headache to confusion, seizures, and coma in severe cases.
Hypernatremia (high sodium, >145 mEq/L) is usually a result of dehydration—not having enough water to balance the sodium. It causes intense thirst, confusion, and muscle twitching.
Potassium Disorders: Hypokalemia and Hyperkalemia
Potassium is vital for heart and muscle function.
Hypokalemia (low potassium, <3.5 mEq/L) can be caused by diuretic use, vomiting, or diarrhea. It leads to muscle weakness, cramps, and potentially dangerous heart arrhythmias.
Hyperkalemia (high potassium, >5.0 mEq/L) is a serious condition most often linked to kidney failure or medications that block potassium excretion (like ACE inhibitors). It can cause muscle weakness, confusion, and life-threatening cardiac arrest.
Calcium Disorders: Hypocalcemia and Hypercalcemia
Calcium is more than just a bone-builder.
Hypocalcemia (low calcium) can cause muscle twitching, spasms, and numbness or tingling in the fingers and toes. It’s often related to parathyroid gland issues or vitamin D deficiency.
Hypercalcemia (high calcium) can lead to “bones, stones, groans, and psychiatric overtones”—bone pain, kidney stones, abdominal pain (constipation), and confusion or fatigue. The most common causes are an overactive parathyroid gland or certain types of cancer.
Magnesium Disorders: Hypomagnesemia and Hypermagnesemia
Magnesium is a cofactor in hundreds of enzymatic reactions.
Hypomagnesemia (low magnesium) is often overlooked but can cause muscle weakness, tremors, and heart arrhythmias. It frequently occurs alongside low calcium and potassium levels. Alcoholism and gastrointestinal disorders are common causes.
Hypermagnesemia (high magnesium) is rare and almost always occurs in the setting of kidney failure and excessive magnesium intake (e.g., from antacids or laxatives). It can cause muscle weakness, low blood pressure, and cardiac arrest.
How Are Electrolyte Imbalances Diagnosed and Treated?
Diagnosing an electrolyte imbalance is a straightforward process that begins with a simple blood test.
What to Expect: Blood Tests (BMP, CMP, Electrolyte Panel)
Your doctor will likely order one of the following tests:
- Electrolyte Panel: This test specifically measures the main electrolytes: sodium, potassium, chloride, and bicarbonate.
- Basic Metabolic Panel (BMP): This common test includes the electrolyte panel plus tests for kidney function (BUN, creatinine) and blood sugar (glucose).
- Comprehensive Metabolic Panel (CMP): This expands on the BMP by adding tests for liver function and proteins.
These tests provide a clear snapshot of your electrolyte levels and help your doctor identify the underlying cause of any imbalance.
Understanding Your Lab Results
Your results will show your electrolyte levels compared to a “normal” reference range.
For example, a normal serum potassium range is typically 3.6 to 5.0 mmol/L.
It’s important to discuss your results with your healthcare provider, as they will interpret them in the context of your overall health and symptoms.
Common Treatment Approaches
Treatment depends entirely on the specific electrolyte, the severity of the imbalance, and its cause.
- For mild deficiencies: Dietary changes or oral supplements may be sufficient. For example, eating a banana for low potassium.
- For severe deficiencies: Intravenous (IV) fluids containing the needed electrolyte are often administered in a hospital setting for rapid and controlled correction.
- For excesses: Treatment may involve IV fluids to dilute the electrolyte and help the kidneys flush it out, medications that promote excretion (like diuretics), or in severe cases of kidney failure, dialysis.
Electrolytes for Special Populations: Who Needs to Pay Extra Attention?
While everyone needs electrolytes, certain groups are at a higher risk for imbalances and have unique needs.
Endurance Athletes: Sweat, Hydration, and Performance
Athletes lose both water and electrolytes—primarily sodium and chloride—through sweat. During long periods of exercise, especially in the heat, these losses can be significant. This can lead to two dangerous conditions:
- Dehydration and Hypernatremia: Losing more water than sodium can concentrate sodium in the blood, leading to high sodium levels.
- Exercise-Associated Hyponatremia (EAH): This occurs when an athlete drinks excessive amounts of plain water, diluting the body’s sodium levels. A study on ultramarathon runners found that EAH is often caused by overconsumption of hypotonic fluids. The safest strategy is to drink to thirst and consider electrolyte-containing sports drinks during events lasting longer than 90 minutes.
The Elderly: Age-Related Risks and Medication Interactions
Older adults are particularly vulnerable to electrolyte imbalances. A review published in Advances in Chronic Kidney Disease highlights several reasons:
- Decreased Kidney Function: Kidney function naturally declines with age, impairing the ability to conserve water and regulate electrolytes.
- Reduced Thirst Sensation: The sense of thirst can diminish, leading to inadequate fluid intake.
- Polypharmacy: Older adults are more likely to be on multiple medications (e.g., diuretics, blood pressure drugs) that can affect electrolyte levels.
Hyponatremia and hypernatremia are the most common and dangerous electrolyte disorders in this population, associated with increased risks of falls, confusion, and mortality.
Individuals with Chronic Conditions
People with certain chronic diseases must carefully monitor their electrolyte balance:
- Chronic Kidney Disease (CKD): Impaired kidneys struggle to excrete potassium, phosphate, and sodium, making hyperkalemia and fluid overload common risks.
- Congestive Heart Failure (CHF): Patients often retain fluid, which can dilute sodium levels (hyponatremia). Additionally, diuretics used to treat CHF cause potassium and magnesium loss.
- Diabetes: High blood sugar can act as an osmotic diuretic, causing fluid and electrolyte loss through frequent urination. Diabetic ketoacidosis is a life-threatening condition involving severe electrolyte disturbances.
How to Get Electrolytes: Diet, Drinks, and Supplements
For most healthy individuals, a balanced diet provides all the electrolytes needed. However, in certain situations, you may need to be more intentional about your intake.
Top Food Sources for Each Key Electrolyte
| Electrolyte | Excellent Food Sources |
|---|---|
| Sodium | Table salt, pickled foods, cheese, processed meats, soups, and condiments. |
| Potassium | Bananas, potatoes, spinach, avocados, beans, lentils, and dried apricots. |
| Calcium | Dairy products (milk, yogurt, cheese), fortified plant milks, leafy greens (kale, collards), and sardines. |
| Magnesium | Nuts (almonds, cashews), seeds (pumpkin, chia), spinach, black beans, and dark chocolate. |
| Chloride | Table salt, seaweed, tomatoes, lettuce, and olives. |
| Phosphate | Meat, poultry, fish, dairy, nuts, and whole grains. |
Sports Drinks vs. Water: When Do You Need Electrolyte Drinks?
The choice between water and an electrolyte drink depends on the duration and intensity of your activity.
- For exercise under 60-90 minutes: Water is usually sufficient for hydration.
- For prolonged or intense exercise (>90 minutes): A sports drink containing carbohydrates (for energy) and electrolytes (to replace sweat losses) can improve performance and prevent imbalances.
- During illness (vomiting/diarrhea): An oral rehydration solution (ORS), which has a specific balance of electrolytes and sugar, is more effective than a typical sports drink for rehydration.
DIY Homemade Electrolyte Drink Recipe
You can easily make your own rehydration drink at home. This simple recipe provides a good balance of fluids, carbohydrates, and electrolytes.
- Start with a base: 4 cups (1 liter) of water.
- Add a pinch of salt: 1/4 teaspoon of table salt (for sodium and chloride).
- Add a sugar source: 2 tablespoons of sugar or honey (for glucose to aid absorption and provide energy).
- Add a potassium source (optional): 1/4 cup of orange juice or a small amount of cream of tartar.
- Flavor it (optional): A squeeze of lemon or lime juice.
Shake well until all ingredients are dissolved. This is a cost-effective alternative for rehydration after moderate activity or during mild illness.
The Role of Supplements: Are They Necessary?
For the vast majority of people, a balanced diet is enough to meet electrolyte needs.
Supplementing without a diagnosed deficiency or a doctor’s recommendation can be dangerous, as it can easily lead to an excess (a “hyper” state), which can be just as harmful as a deficiency.
Always consult a healthcare professional before taking electrolyte supplements.
Frequently Asked Questions (FAQ)
What are the first signs of an electrolyte imbalance?
Early signs often include fatigue, headache, nausea, and muscle cramps or weakness. These symptoms are non-specific, so context (like recent illness or intense exercise) is important.
Can drinking too much water cause an electrolyte imbalance?
Yes. Drinking excessive amounts of plain water, especially during endurance exercise, can dilute sodium levels in your blood, leading to a dangerous condition called hyponatremia.
What is the best drink to restore electrolytes?
For illness-related dehydration, an oral rehydration solution (ORS) is best. For post-exercise recovery after prolonged activity, a sports drink with carbohydrates and sodium is effective. For daily needs, water and a balanced diet suffice.
Do I need electrolytes every day?
Yes, your body requires a daily intake of electrolytes from food and fluids to perform its essential functions. A healthy, varied diet typically provides adequate amounts for most people.
How quickly can you correct an electrolyte imbalance?
This depends on the severity. Mild imbalances can be corrected within hours to days through diet and fluids. Severe imbalances require medical intervention (often IV fluids) and must be corrected slowly and carefully in a hospital to avoid complications.
Are electrolyte powders better than sports drinks?
Powders can be more customizable and cost-effective. They allow you to control the concentration of electrolytes and sugar. However, ready-to-drink sports drinks offer convenience. The “better” option depends on your specific needs and preferences.
Does coffee deplete electrolytes?
Coffee is a mild diuretic, which can increase urine output and a small loss of electrolytes like sodium and potassium. However, for moderate coffee drinkers, the effect is generally minimal and unlikely to cause a significant imbalance.
What electrolyte is most important for muscle cramps?
While cramps can be caused by imbalances in potassium, calcium, or magnesium, dehydration and sodium loss are often primary culprits during exercise. A lack of magnesium is also frequently linked to muscle relaxation issues.
Conclusion
Electrolytes are far more than just an ingredient in sports drinks, they are the microscopic, charged particles that power your body’s most fundamental operations.
From the spark of a nerve impulse to the rhythm of your heart, maintaining a delicate balance of these minerals is essential for your health and well-being.
For most people, a balanced diet rich in fruits, vegetables, and whole foods provides all the electrolytes needed.
However, during intense exercise, illness, or for those with certain medical conditions, paying closer attention to electrolyte intake is critical.
Recognizing the symptoms of an imbalance and knowing when to seek medical advice can prevent serious health complications.
By understanding the roles of sodium, potassium, and their counterparts, you can make informed choices to keep your internal electrical grid running smoothly.
If you have questions or concerns about your electrolyte levels, the best course of action is always to consult with a healthcare professional.

