The global dietary supplements market is a multi-billion dollar industry, built on the promise of better health, enhanced performance, and filling nutritional gaps.
Yet, this promise often masks a troubling reality.
A landmark study published in JAMA revealed that between 2007 and 2016, the U.S. Food and Drug Administration (FDA) identified 776 dietary supplements adulterated with hidden, unapproved pharmaceutical ingredients.
This isn’t a rare occurrence, it’s a systemic problem.
So, are supplements safe? The answer is complicated.
While many products are legitimate, the market is loosely regulated, creating a “wild west” environment where consumers must be their own sheriffs.
This guide will equip you with the knowledge to navigate this landscape, highlighting five critical red flags that signal a product might be ineffective, misleading, or downright dangerous.
In This Article
Are Supplements Safe? Why This Question is a Major Health Concern
Before diving into the red flags, it’s crucial to understand why the supplement industry requires such careful navigation.
The primary issues stem from a significant regulatory gap and the resulting prevalence of contaminated and adulterated products.
The Regulatory Gap: Understanding DSHEA and the FDA’s Role
Unlike prescription drugs, which must undergo rigorous testing for safety and efficacy to gain pre-market approval from the FDA, dietary supplements operate under a different set of rules.
The governing law is the Dietary Supplement Health and Education Act of 1994 (DSHEA).
Under DSHEA, manufacturers are responsible for ensuring their products are safe and that any claims are truthful and not misleading.
However, they are not required to provide evidence of safety or efficacy to the FDA before marketing their products.
The FDA’s role is largely reactive, it can only take action—such as issuing warnings or recalls—after a product is on the market and has been found to be unsafe or misbranded.
This post-market surveillance system places a significant burden on consumers to assess the safety and quality of products themselves. This regulatory gap is the primary reason the question “are supplements safe?” is so critical, as there is no gatekeeper ensuring safety before a supplement hits the shelves.
The Hidden Dangers: Contamination and Adulteration
The lack of pre-market oversight creates opportunities for low-quality and dangerous products to enter the market.
Independent testing frequently uncovers significant problems.
For instance, ConsumerLab.com, a leading third-party tester, has found problems with over 20% of the thousands of supplements it has tested.
These problems range from containing less of an active ingredient than claimed to being contaminated with heavy metals, pesticides, or undeclared pharmaceutical drugs.
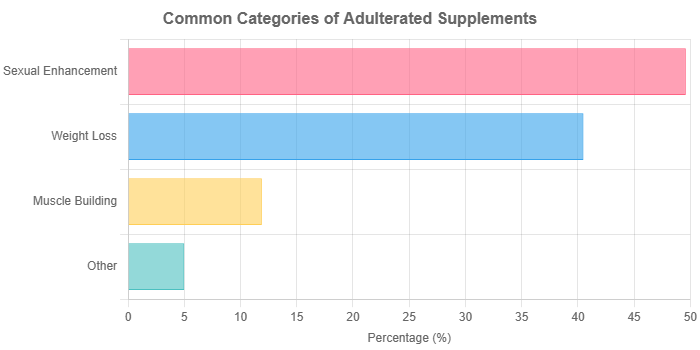
The previously mentioned JAMA study found that products for sexual enhancement, weight loss, and muscle building were the most common culprits for containing hidden drugs like sildenafil (Viagra), sibutramine (a banned weight-loss drug), and synthetic steroids.
Red Flag #1: Are the Health Claims Too Good to Be True?
If a supplement’s promises sound like a magic bullet, your skepticism should be on high alert.
Exaggerated and unsupported claims are a classic sign of a questionable product.
“Cure-Alls” and Disease Treatment Claims
This is the most blatant red flag.
According to FDA regulations, supplements cannot legally claim to diagnose, treat, cure, or prevent any disease.
If you see a product marketed with phrases like “reverses diabetes”, “cures cancer” or “prevents heart disease”, it is making an illegal drug claim and should be avoided.
When you ask, “are supplements safe?”, products making these claims are definitively not.
Legitimate supplements can only make “structure/function” claims, such as “calcium builds strong bones” or “supports a healthy immune system.”
These must be accompanied by the disclaimer: “This statement has not been evaluated by the Food and Drug Administration”.
Vague Promises and Misleading Buzzwords
Manufacturers often use enticing but meaningless terms to sell products. Be wary of:
- “Pharmaceutical Grade”: This term has no official or legal definition in the context of dietary supplements. It’s a marketing buzzword designed to imply higher quality without any verifiable standard.
- “FDA Approved Laboratory”: The FDA does not approve laboratories for supplement testing. While a lab can be “FDA registered”, this is not an endorsement of its methods or results.
- Vague Promises: Terms like “slimming tea”, “detoxifies your body” or “boosts metabolism” are often scientifically unsubstantiated and designed to exploit consumer hopes without making a specific, provable claim.
Red Flag #2: Does the Label Hide More Than It Reveals?
A supplement label should be a source of clear, transparent information.
When it’s confusing or omits key details, it’s often intentional.
The “Proprietary Blend” Trap
A “proprietary blend” or “complex” is a list of ingredients that only discloses the total weight of the blend, not the precise amount of each individual ingredient.
While legal, this practice is a major red flag for several reasons:
- It hides dosages: The most expensive and effective ingredient might be present in only trace amounts, while the bulk of the blend is cheap filler.
- It prevents informed decisions: You cannot know if you are getting a clinically effective dose of any specific ingredient.
- It masks “ingredient dusting”: This is the practice of adding a tiny, ineffective amount of a popular ingredient just to be able to list it on the label.
Always prioritize products that clearly list the exact amount of each active ingredient.
Transparency is a hallmark of a reputable brand.
Misleading Ingredient Quantities
Even when amounts are listed, they can be deceptive.
For example, a label might boast “1.000 mg of Magnesium Citrate”, leading you to believe you’re getting 1.000 mg of magnesium.
In reality, magnesium citrate is only about 11% elemental magnesium, so you’re actually getting around 110 mg.
A trustworthy label will state: “Magnesium (as magnesium citrate) 110 mg.”
The same issue applies to fish oil (look for the specific amounts of EPA and DHA, not just the total fish oil) and herbal extracts (look for standardization to a specific active compound, like “turmeric extract standardized to 95% curcuminoids”).
Red Flag #3: Is the Product Lacking Independent Verification?
Since you can’t always trust the manufacturer’s claims, independent, third-party verification is one of your most powerful tools for ensuring quality and safety.
The Importance of Third-Party Certification
Third-party certification involves an independent organization testing a supplement to verify its quality.
For consumers wondering “are supplements safe?”, this is one of the most important signs of a trustworthy product.
These tests typically confirm that:
- The product contains the ingredients listed on the label in the declared amounts.
- It does not contain harmful levels of contaminants like heavy metals, pesticides or microbes.
- It is manufactured according to the FDA’s Good Manufacturing Practices (GMPs).
Crucially, these certifications do not guarantee that a product is effective.
They only verify its contents and purity.
A claim of “third-party tested” without naming the certifying body is meaningless.
Seals to Look For: NSF, USP, and ConsumerLab
Look for official seals from reputable organizations on the product packaging.
The most recognized in the U.S. are:
- NSF International: Offers several certifications, including “NSF Certified for Sport”, which also tests for over 270 substances banned by major athletic organizations.
- U.S. Pharmacopeia (USP): The USP Verified mark indicates the product meets stringent standards for identity, potency, purity, and performance.
- ConsumerLab.com: While they have an approval seal for products that pass their testing, they are primarily known for their published reports that compare and review products.
| Organization | What It Verifies | Key Features |
|---|---|---|
| USP (U.S. Pharmacopeia) | Identity, Potency, Purity, Performance | Sets official public standards, verifies that the supplement will break down and release into the body within a specified time. |
| NSF International | Identity, Potency, Purity, Contaminants | Conducts annual audits of manufacturing facilities. The “Certified for Sport” program is a gold standard for athletes. |
| ConsumerLab.com | Identity, Potency, Purity | An independent testing company that publishes results on which products pass or fail. Offers an “Approved Quality” seal. |
Red Flag #4: Does the Marketing Rely on Hype Over Science?
Reputable companies back their products with science.
Disreputable ones rely on emotional appeals and pseudoscience.
Personal Testimonials vs. Clinical Evidence
Marketing that heavily features dramatic personal testimonials (“I lost 50 pounds in two months!”) instead of citing peer-reviewed scientific studies is a major red flag.
Testimonials are anecdotal, impossible to verify, and often fabricated or paid for.
Real evidence comes from well-designed clinical trials published in reputable scientific journals like those found on PubMed.
The “All-Natural” Fallacy
The word “natural” is one of the most abused terms in marketing.
It evokes a sense of safety, but it is not synonymous with “safe”.
This is a critical point when asking “are supplements safe?”.
Many natural substances are toxic.
Arsenic, lead, and hemlock are all 100% natural.
Furthermore, some “natural” herbal supplements, like comfrey and kava, have been linked to severe liver damage, as noted by the National Center for Complementary and Integrative Health (NCCIH).
The safety of a supplement depends on its chemical composition, dosage, and preparation, not its origin.
Red Flag #5: Are You Buying from a High-Risk Category or Source?
Some product categories and sales channels are inherently riskier than others and require extra vigilance.
The “Big Three” Risky Categories
As shown in the chart above, research consistently shows that supplements marketed for the following purposes are most likely to be adulterated with hidden drugs:
- Weight Loss: Often contain banned stimulants, diuretics, or antidepressants.
- Sexual Enhancement: Frequently spiked with prescription erectile dysfunction drugs or their unapproved chemical analogs.
- Bodybuilding/Athletic Performance: May contain anabolic steroids or other dangerous stimulants.
If you are considering a supplement in one of these categories, third-party certification is not just recommended—it’s essential.
The Wild West of Online Marketplaces
While convenient, large online marketplaces like Amazon can be a hotbed for counterfeit and low-quality supplements.
Unscrupulous sellers can easily set up shop, sell products from unknown brands, and disappear when problems arise.
Be particularly cautious of products with no established brand history, thousands of generic-sounding positive reviews appearing in a short time, and prices that are significantly lower than established competitors.
How Can You Choose Safe and Effective Supplements?
Navigating the market successfully and answering the question “are supplements safe?” for yourself comes down to a simple, four-step process.
Step 1: Consult Your Healthcare Provider
Before taking any new supplement, talk to your doctor, pharmacist, or a registered dietitian.
They can help you determine if you actually need the supplement, recommend an appropriate dosage, and check for potential interactions with any medications you are taking.
Step 2: Research the Specific Ingredient and Dosage
Use reliable, evidence-based resources like the Office of Dietary Supplements (ODS) at the NIH or Examine.com to research the specific supplement you’re considering.
Find out what the scientific evidence says about its effectiveness and what dosages were used in clinical studies.
Step 3: Vet the Brand and Look for Third-Party Seals
Choose brands that are transparent about their manufacturing processes and sourcing.
Most importantly, select products that bear a third-party certification seal from USP, NSF, or another reputable organization.
Step 4: Check the FDA’s Tainted Supplements Database
The FDA maintains a public database of products found to contain hidden ingredients.
Before buying, it’s wise to do a quick search on the FDA’s Tainted Products page to see if the product or brand has been flagged.
Frequently Asked Questions (FAQ)
Are supplements regulated by the FDA?
Yes, but differently and less strictly than drugs. The FDA regulates them under the DSHEA of 1994, which does not require pre-market approval for safety or efficacy. The FDA’s role is primarily to take action against unsafe products after they are already on the market.
What does “third-party certified” really mean?
It means an independent organization has tested the product to verify that it contains what the label claims, is free from harmful contaminants, and was manufactured properly. For consumers asking “are supplements safe?”, this certification is a key indicator of quality, but it does not guarantee the product is effective.
Can I trust reviews on Amazon for supplements?
Be very cautious. While some reviews are genuine, the platform is susceptible to fake reviews. It’s safer to rely on expert analysis and third-party testing rather than user reviews, especially for unknown brands.
Is a more expensive supplement better?
Not necessarily. Price is not a reliable indicator of quality. Independent testing often finds that affordable, store-brand products that are third-party certified perform just as well or better than expensive, boutique brands.
What’s the danger of a “proprietary blend”?
The main danger is that you don’t know the dose of any specific ingredient. This makes it impossible to know if you’re getting an effective amount or if you’re taking too much of something. It often serves to hide an ineffective formula.
How do I report a bad reaction to a supplement?
You should first inform your healthcare provider. You can also report it directly to the FDA through their Safety Reporting Portal (MedWatch). This helps the agency track and identify dangerous products.
Do I need to take a multivitamin?
Most people can get all the necessary nutrients from a balanced diet. However, some populations (like pregnant women or those with specific deficiencies) may benefit. A 2024 NIH study found no association with lower risk of death for healthy adults. Always consult a doctor first.
Can supplements interact with my medication?
Yes, and it can be very dangerous. This is one of the most important factors when considering if supplements are safe for you. For example, Vitamin K can reduce the effectiveness of blood thinners like warfarin, and St. John’s Wort can interfere with antidepressants and birth control pills. Always discuss supplements with your doctor or pharmacist.
Conclusion
The dietary supplement industry is not going to be overhauled overnight.
For the foreseeable future, the responsibility for answering the question “are supplements safe?” will remain squarely on the consumer’s shoulders.
By learning to spot these five red flags—unrealistic claims, opaque labels, a lack of independent verification, hype-based marketing, and high-risk categories—you can build a powerful shield against dangerous and fraudulent products.
Your health is too important to leave to chance or to a manufacturer’s unverified promises.
Arm yourself with knowledge, adopt a healthy dose of skepticism, and always follow the golden rule of supplement safety: Consult, Verify, and Question.

